Images and/or pictures can explain far better than our several pages of detail life
history of the standard document, and most importantly we want you to visualize
your business and compare. You can make justification about your business goals
and success;
Did you know that, calibration and repair service in North America and Europe is
anticipated to reach $3.98 billion by 2022.
If you're looking to expand your calibration capabilities, improve customer business
relations, provide in-house training to your employees and measure "Return On Investments"
(ROI), then CAL LAB ACCESS, LLC would like to provide you the cost effective solutions
when you need them.
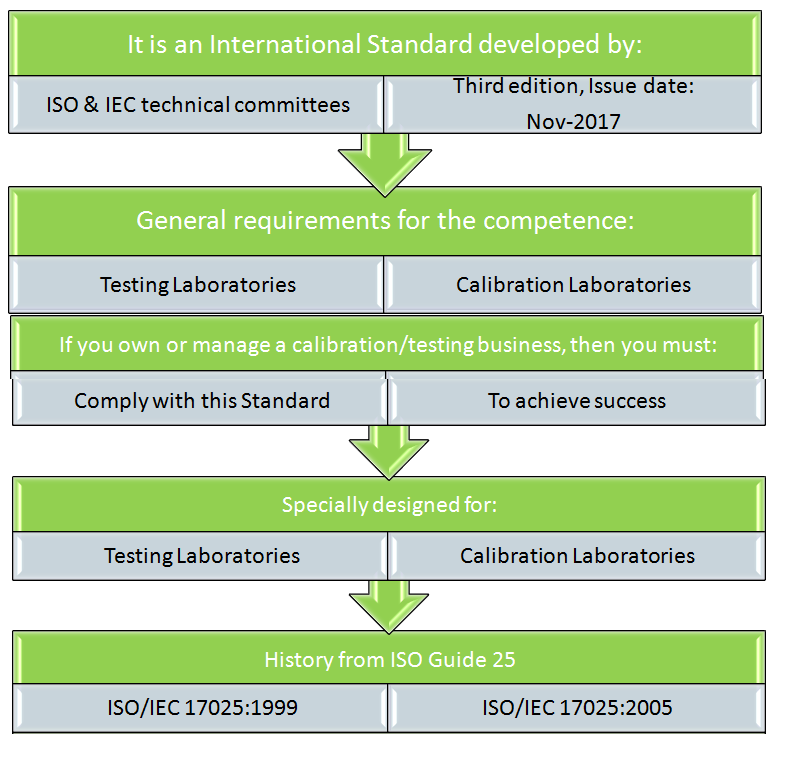
What is it?
ISO/IEC 17025:2017, it is an international standard developed and maintained by
International Standard Organization (ISO) and Inter-Technical Committee (IEC). For
this very particular reason this standard is always written as ISO/IEC 17025.
Why should I adopt?
Who is asking?
What are the benefits?
Where can I find information?
CAL LAB ACCESS, LLC is in business to assist and help calibration and testing laboratories
around the globe with complete laboratory management solutions. Our services include
but not limited to;
- Calibration / testing laboratory setup
- Asset Management Database (dynamic) with
- No software needed, no licensing fee, no upfront fee, no setup
fee
- Complete quality management system per ISO/IEC 17025 standard
- Complete quality management system per ISO 9001 standard (available)
- Unlimited access to all your employees
- Unlimited access to all your customers
- Unlimited access to all your suppliers
- Manage all your employee’s technical competency
- Manage and track calibration process
- Manage quotations
- Manage documentation
- Calibration templates with uncertainty calculations
- Audit checklist
- Intermediate checks
- Proficiency testing plans
- Access control
- Technical support
- Much more features are being added on day to day basis
How can I achieve?
Our team of experts work with you to provide the project details and review your
laboratory’s technical capabilities for accreditation purposes. we were able to
complete the accreditation processes for other laboratories within three (3) to
six (6) months.
Looking forward to providing you an excellent consulting service.